Advancing Therapeutics Targeting Cytokines
- Cytokine and interleukin receptor assays
- Ready-to-use, cell-based bioassays
- Signaling pathway reporter assays
Functional, Cell-based Assays for Analyzing Cytokine Signaling
As key mediators of immune responses, cytokines have emerged as major drug targets for inflammatory and autoimmune diseases. A significant number of cytokine-based clinical and preclinical development trials have surfaced in the last decade, with many approved drugs targeting inflammatory cytokines such as Humira® (TNFα), Dupixent® (IL-4 & IL-13), Stelara® (p40 of IL-12 and IL-23), Tremfya® (IL-23), and Actemra® (IL-6),* while several others are under development.
Advances in elucidating diverse cytokine signaling pathways mediated by different receptor combinations and a range of targets have provided a greater understanding of cytokine functions and signaling. Yet, the characterization of candidate therapeutics involved in complex cytokine signaling pathways presents challenges and requires unique and specific tools. Eurofins DiscoverX® has a wide array of cell-based assays designed to measure signaling from various targets with a primary focus on accelerating discovery and development times for cytokine therapeutics for a number of conditions including inflammatory and autoimmune diseases.
Cytokine Receptor Dimerization and Signaling Reporter Assays
Therapeutics aimed at inflammatory cytokines act either by stimulating or inhibiting cytokine signaling pathways. Two assay formats offered by Eurofins DiscoverX relevant to cytokine signaling are receptor dimerization and reporter assays. Cytokine induced signaling via dimerization and reporter assays based on the application of EFC technology are shown in Figure 1.
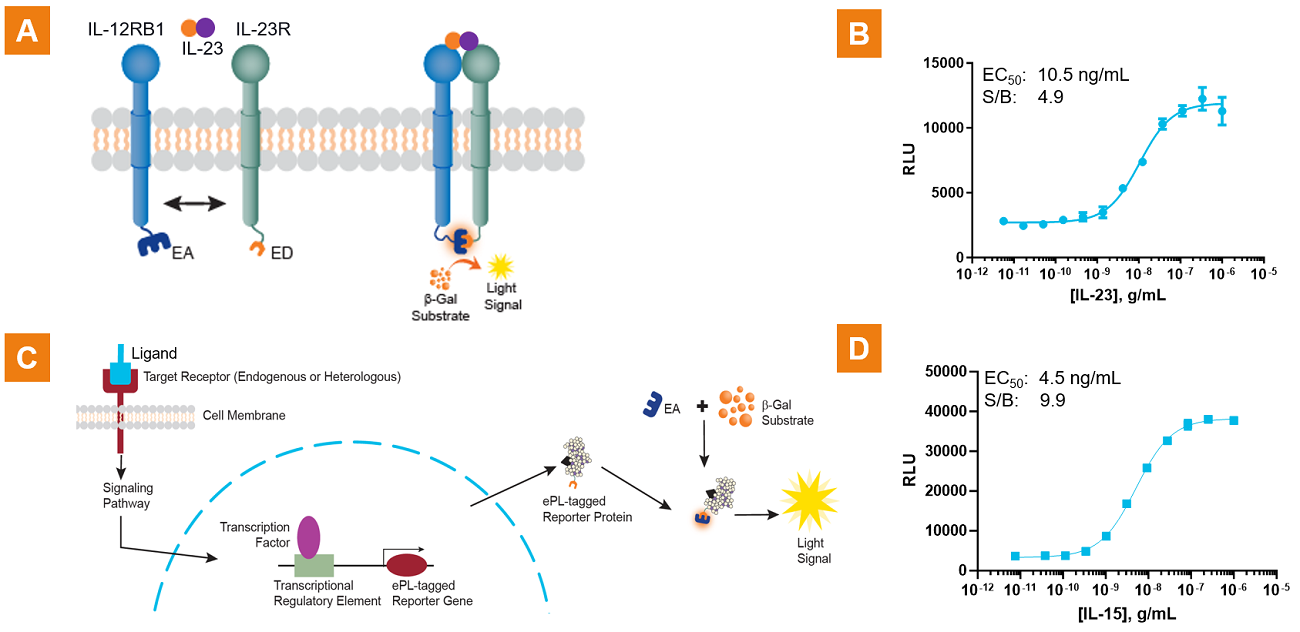
Figure 1. IL-23 induced receptor dimerization assay and signaling reporter assay. A. and B. demonstrates the quantitation of receptor dimerization and gain of signal response using a PathHunter® IL-23 dimerization cell-based assay. IL-23 receptor subunits are tagged with EFC β-galactosidase (β-gal) fragments EA (enzyme acceptor) and ED (enzyme donor). Upon agonist treatment, the two subunits dimerize, resulting in the complementation of the two β-gal fragments to create a functional β-gal enzyme, whose activity is measured with a luminometer. A quantitative gain-of-signal sigmoidal response as a measure of IL-23 receptor dimerization is observed. C. and D. show cytokine activation measured by quantifying a distal receptor signaling event (transcriptional activation) using an EFC assay format, wherein cytokine binding to the receptor initiates a signaling cascade resulting in ED-tagged reporter protein production and EA fragment complementation with the ED fragment upon cell lysis (C.). A quantitative gain-of-signal sigmoidal response as a measure of IL-15 signaling is observed (D.).
Both dimerization and reporter assays can be used for measuring signaling pathways. Dimerization assays are more specific in their cytokine activation, while reporter assays often show larger assay windows as well as good sensitivity. An example of dimerization assay specificity is observed when different cytokines (IL-1β, IL-33, TNFα, etc.) are tested; only IL-1β results in IL-1R1/IL-1RAP dimerization. In contrast, when an NF-kβ signaling reporter assay is used to measure cytokine activation signaling, a number of cytokines (TNFα, CD40L) in addition to IL-1β activate NF-kβ signaling (data not shown). However, the signal/background (S/B) window observed for IL-1β is much larger with the NF-kꞵ signaling reporter asay (S/B = 58.3, EC50 = 1.4 ng/mL) compared to that observed with the IL-1/IL-1RA dimerization assay (S/B = 5.7, EC50 = 0.83 ng/mL).
Specificity of Receptor Dimerization Assays
Greater specificity of receptor dimerization assays is further demonstrated with two members of the IL-12 family, IL-12 and IL-23 cytokines, both validated therapeutic targets for Crohn’s disease, plaque psoriasis, and psoriatric arthritis. IL-12 and IL-23 are both heterodimers containing the common cytokine subunit (p40) that binds to the IL-12Rβ1 receptor subunit. Their unique cytokine subunits (p35 and p19 for IL-12 and IL-23, respectively) and receptor partners (IL-12Rβ2 and IL-23R for IL-12 and IL-23, respectively) are responsible for specific signaling pathway activation. When IL-12, IL-23, and a homodimer of the p40 subunit are used in the assays (see Figures 2. B.), only IL-12 activates IL-12Rβ1/IL-12Rβ2 dimerization and not IL-23R/IL-12Rβ1 dimerization. Similarly, only IL-23 activates IL-12Rβ1/IL-23R (see Figure 2. C). The p40 subunit monomer of IL-12 or IL-23 results in equally poor activation in both assays as expected (data not shown).
When using a therapeutic antibody (Ustekinumab marketed as Stelara®) targeting the common p40 subunit found in IL-12 and IL-23, the antibody inhibits both IL-12R and IL-23R dimerization (see the blue curves in Figures 2. B. and C.). However, antibodies (Guselkumab (Tremfya) and Riazankizumab (Skyrizi)) targeting the p19 subunit specific for IL-23 result in an antagonistic IL-23 signaling in the IL-23R dimerization assay, but have no effect on IL-12 activation of IL-12 receptor dimerization. The data with these therapeutic antibodies demonstrate how the assays can be used to compare the activity and potency of different drugs.
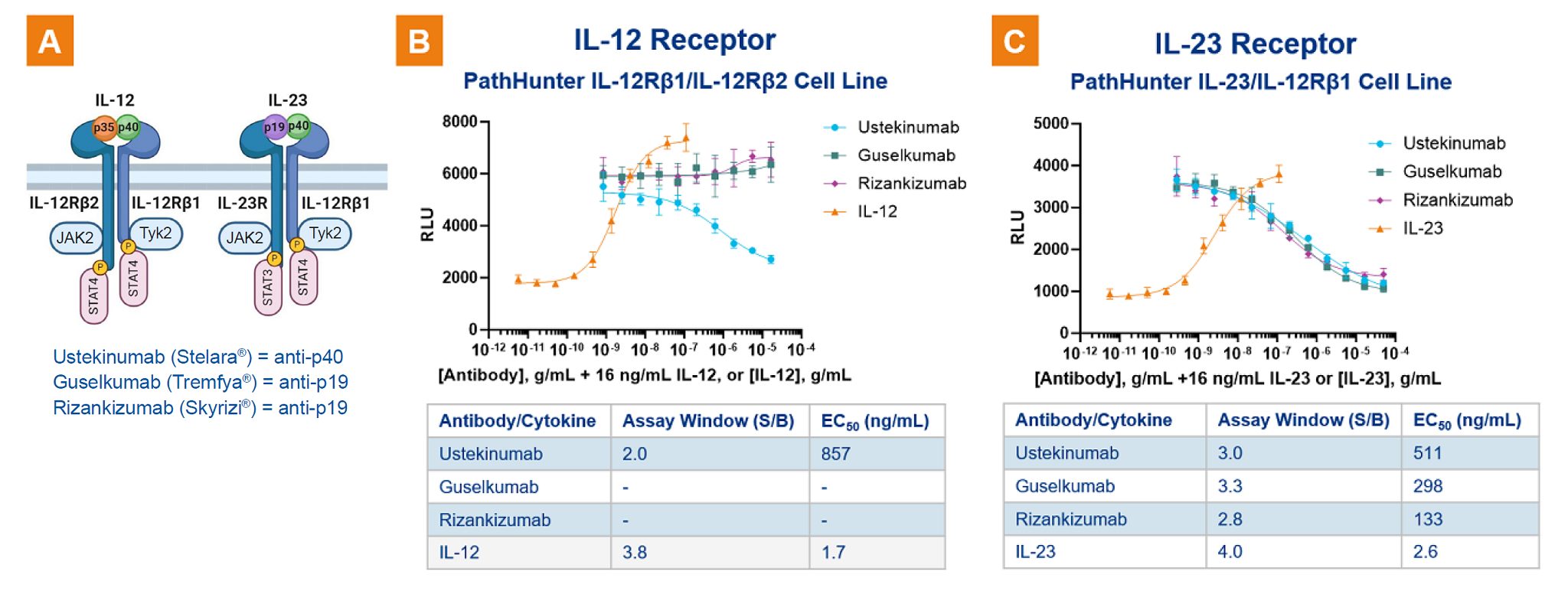
Figure 2. Therapeutics targeting p40 subunit of IL-23 and IL-12. The effects of antibodies (anti-p40 and anti-p19) on IL-12 and IL-23 receptor dimerization are shown wherein Ustekinumab targeting the common p40 subunit inhibits dimerization of IL-12Rβ1 to both IL-12Rβ2 and IL-23R. Anti-p19 antibodies however only inhibit IL-23 signaling in the IL-23 receptor dimerization assay and have no effect in the IL-12 receptor dimerization assay.
Sensitivity of Signaling Reporter Assays
An NF-kB reporter assay was used to compare IL-1β agonist and antagonist responses and sensitivities to the IL-1RA/IL-1RAP dimerization assay. IL-1β activation (agonist), and its inhibition (in antagonist mode with anti-IL-1β antibody) was measured with or without 5% normal human serum (NHS). The S/B ratios obtained with the reporter assay format were larger in both agonist and antagonist mode, but this reporter assay sensitivity was attenuated in antagonist mode, particularly in the presence of 5% NHS (Figure 3. A.). When using the dimerization assay for IL-1β signaling, there was no change in the assay window observed or the IC50 with anti-IL-1β drug in the presence of 10% NHS (Figure 3. B.).
This example demonstrates that the signaling reporter assay format results in a greater response to IL-1β activation than the dimerization assay, even in the presence of NHS. However, the reporter assay exhibits a susceptibility to changes in S/B or potency in the presence of human serum since components in the serum may affect other signaling pathways in the cell or other steps in the IL-1β pathway downstream of dimerization, impacting NF-kB mediated transcription.
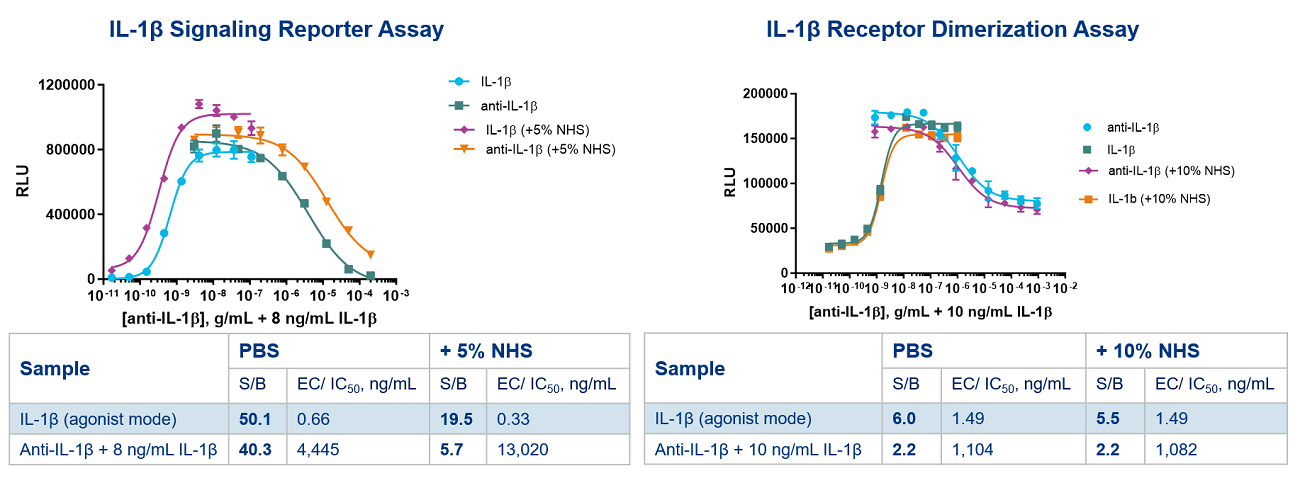
Figure 3. IL-1βR signaling reporter and receptor dimerization assays. The signaling reporter assay used to measure IL-1β signaling shows attenuated assay windows and increased IC50 in the presence of NHS, while the dimerization assay results are similar in the presence or absence of NHS.
The receptor dimerization assay, as shown in this example, produces robust agonist and antagonist responses in the presence of 10% NHS, which is required for quantifying the presence of anti-drug neutralizing antibodies (NAb) in human serum samples. Hence, dimerization assays are better suited for NAb screening as agonist and antagonist responses exhibit little change when human serum is present.
Summary
Eurofins DiscoverX’s cytokine assays are available in multiple formats to measure receptor activation and dimerization as well as downstream signaling. PathHunter® receptor dimerization assays show greater specificity over reporter assays and are suitable for NAb testing due to their insensitivity in the presence of human serum. PathHunter reporter assays enable the measurement of changes in cytokine signaling and often provide larger assay windows and greater sensitivity for screening.
PathHunter cytokine receptor assays aid in cytokine drug discovery and development:
- Offer mechanism of action (MOA)-based dimerization, signaling reporter, and other assay types covering over 80% of interleukins
- Available in stable cell line, including working cell banks, as well as thaw-and-use eXpress™ and bioassay kit formats with many bioassays qualified with a reference ligand or therapeutic
- Used from discovery through development and into commercial release and stability QC lot testing
Visit DiscoverX.com/interleukins to access functional cell-based assays for over 80% of human interleukins, including for IL-1, IL-2, IL-6, IL-12, IL-7, IL-15, TNFα, GM-CSF, and more.
Resources
- Cytokine and interleukin receptor assays
- Ready-to-use, cell-based bioassays for comparability and QC lot release testing
- Functional cell-based assays to support COVID-19 drug discovery through QC lot release programs
- Signaling pathway reporter assays for understanding therapeutic MOAs
- EFC platform for interrogating biomolecular reactions
* Humira is a registered trademark of AbbVie Inc. Actemra is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of Roche Group; Dupixent is a registered trademark of Sanofi Biotechnology; Stelara is a registered trademark of Johnson & Johnson.
