Functional Assays for Screening, Characterization, & Clinical Development of Agonistic Antibodies for Immunotherapy & Autoimmune Diseases
Agonistic Antibodies and the Role of Checkpoint Receptors in Immune Function
Eurofins DiscoverX® has developed assays for screening, characterization, and clinical development of agonistic antibodies for immunotherapy and autoimmune diseases. Cancer immune therapeutics primarily consist of antagonist antibodies that block inhibitory checkpoint receptors such as PD-1 receptor, but there are a growing number of agonist antibodies targeting co-stimulatory checkpoint receptors under development through several clinical trials. Many are also developed as combination therapy along with checkpoint inhibitors, such as PD-1.
Agonistic antibodies can target both types of checkpoint receptors that allow for immune cell (T-cell) activation. By targeting co-stimulatory checkpoint receptors (e.g., OX40), the signaling pathway involving T-cell receptor (TCR) signaling leading to an anti-tumor response is achieved. On the other hand, by targeting co-inhibitory checkpoint receptor signaling, the agonistic antibodies can suppress T-cell activation that prevents exaggerated immune responses leading to autoimmune disease.
Due to their ability to regulate immune function, agonist antibodies have emerged as an essential class of therapeutics targeting both co-stimulatory and co-inhibitory receptors. Figure 1 illustrates the roles of agonistic and blocking antibodies as they interact with co-stimulatory and co-inhibitory receptors, respectively.
![]()
Figure 1. Co-stimulatory and Co-inhibitory Receptors Involved in T-cell Activation. Agonistic antibodies activate co-stimulatory checkpoint receptors such as CD137, OX40, while blocking antibodies activate co-inhibitory receptors such as PD-1, CTLA-4, etc.
Cell-based Checkpoint Receptor Assays
The vast portfolio of checkpoint receptor assays offered by Eurofins DiscoverX have broad applications for cell-based screening, functional characterization studies, and QC-lot release assays. They can be implemented in several phase-appropriate formats for assessing biologics and small molecule therapeutics.
Eurofins DiscoverX’s proprietary enzyme fragment complementation (EFC) platform described here has provided the best solution for screening and characterizing agonistic antibodies against checkpoint receptor targets (see Figure 2.).
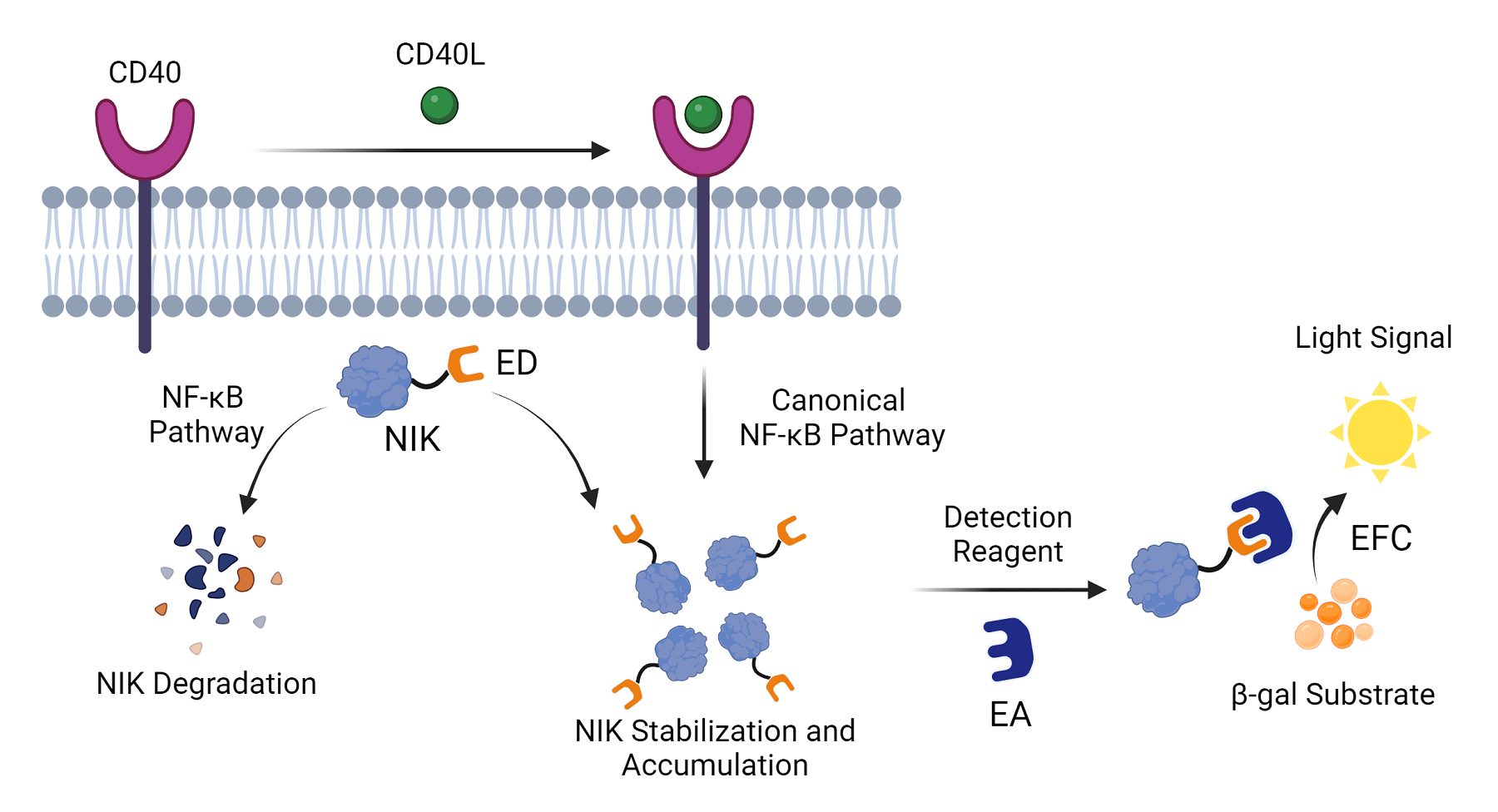
Figure 2. Enzyme Fragment Complementation Assay Design with CD40 Checkpoint Receptor. An EFC-based assay for CD40 co-stimulatory checkpoint receptor. When cells expressing CD40 receptors are not stimulated by a ligand, there is degradation of the cytosolic CD40 signaling protein, NIK, which occurs via the non-canonical NF-κB pathway. However, when a CD40 ligand (CD40L) interacts with the CD40 receptor, the canonical NF-κB pathway is activated, resulting in NIK stabilization and accumulation. In the assay, the NIK protein has been tagged with an enzyme donor (ED), one of the two β-galactosidase (β-gal) enzyme fragments. The addition of a detection reagent containing the other enzyme fragment, the enzyme acceptor (EA), enables the coupling of the two enzyme fragments, resulting in an active β-gal enzyme that causes its substrate to produce a quantitative chemiluminescence signal that can be read in a luminometer.
Cell-based Assay Design for Characterizing Checkpoint Therapeutics – Critical Role of FcγR Receptors
Standard assays developed to evaluate the agonistic activity of antibodies (involving antibodies directed at target cells expressing antigens) have been unsatisfactory with low to modest activities, making them unsuitable for further characterization. An appropriate assay design is hence essential for capturing the agonistic potential of any antibody therapeutic candidate.
One of the critical mechanisms by which therapeutic antibodies elicit clinical efficacy is via activating immune effector components to induce cell death. This is triggered when Fc regions of antibodies bind with Fc receptors (FcγR) expressed on leukocytes. Eurofins DiscoverX has modified the standard assay design for evaluating agonist antibody activity by including FcγR expressing cells as part of the assay design using this mechanism. These FcγR cells do not trigger any signaling, but only function as scaffolds that cluster and better present the antibody to the target cells. This, in turn, enhances the signaling and results in augmented agonism of the antibody. Hence, including cells expressing Fcγ receptors in the assay design can significantly affect the assay outcome. As shown in Figure 3, adding FcγRIIb cells to the assay design along with anti-CD137 antibody caused an increase in the EMAX of the antibody by over 300% compared to antibody alone. A similar response was observed with the anti-CD40 antibody in combination with FcγR cells showing >200% in EMAX.
![]()
Figure 3. Modified Assay Design for Augmenting Agonistic Antibodies and Enhanced Agonism of anti-CD137 and anti-CD40 Antibodies. A. A standard assay design was modified to include FCyR cells. The clustering of cells resulted in capturing an augmented agonistic response of the antibody upon the addition of an appropriate detection reagent. When FcγR cells were added along with anti-CD137 (B.) or anti-CD40 antibodies (C.) in separate assays, enhanced agonistic activity of antibodies were observed compared to no FcγR cells or IgG controls.
FcγR clustering cells have also been shown to augment agonistic antibody potency and efficacy, as observed using assay designs incorporating FcγR cells for evaluating anti-OX40 and anti-TWEAK receptor antibody agonism. However, not every co-stimulatory checkpoint receptor tested showed an increased agonistic antibody response. Examples include CD28 and ICOS. (Data not shown). In vivo studies have also demonstrated that knocking out the expression of Fcγ receptors in mouse models abolishes any observed agonistic effect by the candidate antibody, thereby establishing the significance and physiological relevance of including these cells in the assay design. Lastly, this improved assay design that includes FcγR cells allows rank ordering of potencies when profiling antibody drug candidates and differentiating between their efficacies.
Developing Antibodies for Co-inhibitory Checkpoint Receptors
Using cell-based assays for characterizing agonistic antibodies for co-stimulatory receptors has created momentum to leverage antibodies against co-inhibitory checkpoint targets, particularly as potential therapeutic options against auto-immune diseases.
As an example, the agonistic potential of antibodies against two popular co-inhibitory checkpoint receptor targets – BTLA and PD-1, using this modified assay design was evaluated. Using Eurofins DiscoverX’s BTLA and PD-1 signaling assays, agonistic activities of anti-BTLA and anti-PD-1 antibodies were evaluated, respectively. When evaluating the antibodies alone on specific target cells, marginal agonistic responses were recorded. However, enhanced agonistic responses were observed when FcγR clustering cells were included in the assay design (along with antibodies) (See Figure 4.). While the EMAX for anti-BTLA+ FcγR cells was observed to be enhanced by about 200% compared to antibody alone, the EMAX for anti-PD-1+ FcγR cells using the PD-1 signaling assay was augmented by over 250%.
![]()
Figure 4. Evaluating Agonistic Activity of Anti-BTLA & Anti-PD-1 Antibodies. A. Anti-BTLA antibody clones showing enhanced agonistic activities compared to controls (IgGs and without FcvR cells). B. Anti-PD-1 antibody (Pembrolizumab) showing enhanced agonistic activity compared to controls (IgGs and without FcγR cells).
The use of an optimized assay design as described above can serve as tools to better assess the agonistic potential of an antibody-drug candidate. This data further shows that incorporating FcγR cells in the assay design forms a critical part of testing the agonistic activity of the antibody drug candidate; even though the antibody in question may not have been developed as an agonistic antibody.
Summary
Checkpoint receptors play a critical role in the development and progression of cancer and autoimmune diseases. FcγR clustering augments agonistic activity of checkpoint antibodies, which has significant implications in the characterization and development of therapeutics for cancer and autoimmune disease. Clinical development of agonistic antibodies targeting co-inhibitor checkpoint receptors using FcγR clustering cells offers a new avenue to design therapeutics against auto-immune diseases. Eurofins DiscoverX assays can efficiently characterize agonistic antibodies for immune checkpoint activators and inhibitors. In addition, several cell lines (human and mouse) that over-express distinct types of FcγR are available to determine the best affinity with antibody therapeutic.
Resources & Products
- Webinar: Functional Assays for the Development of Agonistic Abs for Immunotherapies
- White paper: Accelerating Immune Checkpoint Drug Discovery through Functional Cell-Based Assays
- Poster: Ready-to-Use Cell-Based Assays to Enable Immunotherapy Drug Development for checkpoint Receptors
- Checkpoint Receptor Assays & Immuno-oncology Product Solutions
