Evaluate GLP-1R Targeted Therapeutics Using First-to-Market Semaglutide Qualified GLP-1R Bioassay
Glucagon-Like Peptide-1 (GLP-1), a member of the glucagon-secretin peptide family, is secreted from L-cells of the small intestine and binds to GLP-1 receptor (GLP-1R), a class B G Protein-Coupled Receptor (GPCR). GLP-1R is expressed in pancreatic cells and represents an important therapeutic target for Type 2 Diabetes Mellitus (T2DM) as it helps secrete insulin via increased cAMP levels when bound to GLP-1. GLP-1R agonists (also known as incretin mimetics or GLP-1 analogs) are a class of drugs used to treat Type 2 diabetes and obesity. This class of drugs are structurally classified into exendin-4 backbone agents – Exenatide & Lixisenatide (short-acting GLP-1R agonists); and human GLP-1 backbone agents – Liraglutide, Albiglutide, Dulaglutide, and Semaglutide (long-acting GLP-1R agonists).
The increasing global prevalence of T2DM and obesity has advanced the discovery and development of novel therapeutics targeting T2DM and obesity. In recent years, the focus on therapeutics for T2DM has shifted towards the development of not only glucose-lowering drugs, but also those that can reduce body weight. Drugs such as Liraglutide and Semaglutide have been approved as glucose-lowering drug injectables for the treatment of T2DM in adults, in addition to reducing body weight, the latter reportedly causing at least 10% body weight reduction in some patient trials. These agents act by activating GLP-1Rs in the pancreas, which leads to enhanced insulin release and reduced glucagon release-responses that are both glucose-dependent with a consequent low risk for hypoglycemia.
Advantages of Eurofins DiscoverX Cell-Based Assays
Several assays and technologies exist to evaluate cAMP accumulation due to GLP-1 agonist binding to the GLP-1R. These include, but not limited to, ELISA, reporter gene, and TR-FRET assays. However, these assays are labor intensive/tedious to perform, exhibiting short signal stability, and may not be truly reflective of the mechanism-of-action (MOA) that the therapeutic molecule needs to demonstrate.
Eurofins DiscoverX provides a comprehensive portfolio of cell-based assays for glucose metabolism drug discovery and development targeting insulin, IGF-1, GLP-1, and GLP-2 (Figure 1.). These assays are homogeneous, MOA reflective , robust, fast, and simple to run. The assays are available as ready-to-use (RTU) target-based or qualified bioassay kits. The bioassay kits are fit-for-purpose with acceptable levels of linearity, accuracy, repeatability, reproducibility, and precision per industry standards.
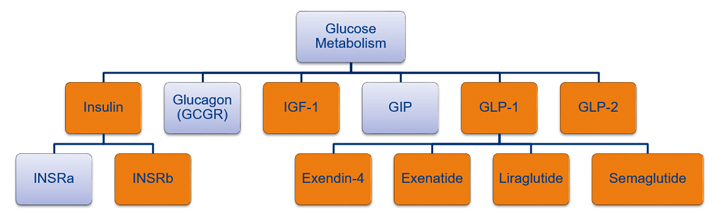
Figure 1. Comprehensive portfolio of GPCR products for glucose metabolism. This illustration shows the Eurofins DiscoverX products available for glucose metabolism targets with orange targets available in bioassay kit format.
cAMP Hunter Assay for Measuring GLP-1R Agonist’s MOA
The cAMP G protein-dependent pathway involves a heterotrimeric (α/β/γ) G-protein containing a GDP molecule bound to the Gα subunit that holds the trimer together. Upon activation, GDP is exchanged for GTP, leading to the dissociation of the Gβ/Gγ dimer from Gα. Both parts remain anchored to the membrane and become free to act upon their downstream effectors and initiate unique intracellular signaling responses. The activated Gα subunit interacts with and regulates many effector molecules such as adenylyl cyclase that can ultimately lead to the accumulation of cAMP.
With the recent approval of Semaglutide as a treatment drug for T2DM, Eurofins DiscoverX has developed a GLP-1R bioassay kit qualified with Semaglutide. The cAMP Hunter™ Semaglutide GLP-1R Bioassay Kit is the first-to-market, plate-based MOA-reflective bioassay kit that is qualified with Semaglutide and measures cAMP accumulation.
cAMP accumulation assays (Figure 2.) are competitive immunoassays amenable for high-throughput screening and utilizes the Enzyme Fragment Complementation (EFC) detection technology (discoverx.com/efc) where a small fragment of ß-galactosidase (ß-gal) enzyme, referred to as enzyme donor (ED) is conjugated with cAMP. This ED-cAMP conjugate and cellular cAMP compete for binding to an anti-cAMP antibody (Ab). With low levels of cellular cAMP, most of the ED-cAMP binds to the cAMP Ab, making the ED-cAMP unable to complement with the added ß-gal larger enzyme acceptor (EA). With high levels of cellular cAMP, the anti-cAMP antibody becomes saturated, allowing the ED-cAMP complex to complement the EA fragment and form an active ß-gal enzyme. The active enzyme subsequently hydrolyzes an added substrate to produce a chemiluminescent detection signal that is directly proportional to the amount of cAMP in the cells.
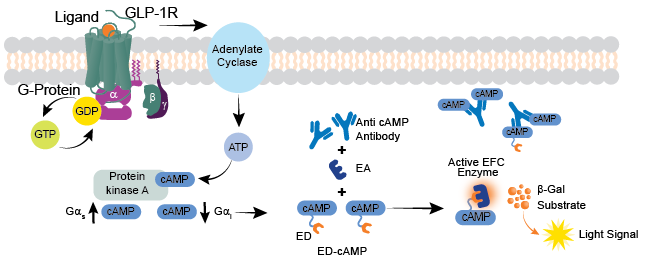
Figure 2. cAMP Hunter GLP-1R Bioassay Principle. cAMP Hunter Semaglutide GLP-1R Bioassay Kit uses the HitHunter™ cAMP assay to detect cAMP accumulation. This assay is a competitive immunoassay, where free cAMP from cell lysates competes for antibody binding against labeled cAMP (ED-cAMP conjugate). Unbound ED-cAMP is free to complement EA to form an active enzyme by EFC that hydrolyzes its substrate to produce a light signal that can be measured. A positive signal is directly proportional to the amount of cellular cAMP.
Semaglutide Bioassay Qualification
The cAMP Hunter Semaglutide GLP-1R Bioassay Kit is a qualified RTU bioassay that is fit-for-purpose for relative potency (RP) applications in QC lot-release testing. The GLP-1R Bioassay was qualified with the drug Ozempic® (a brand name of Semaglutide from Novo Nordisk A/S) over a nominal concentration (NC) range of 50%-150% with 4 runs each NC (Figure 3. A. and B.), and intermediate presicion was evaluated using two analyts over multiple days and two lots of bioassay cells. Figure 3. C. displays the average RP for each NC plotted relative to the expected value to demonstrate dilutional linearity of the assay. The table in Figure 3. C. summarizes the relevant assay characteristics from qualifications of the method. Overall, the qualification study showed excellent accuracy, repeatability, intermediate precision, and excellent dilutional linearity (R2=0.999) for the Semaglutide GLP-1R bioassay. Intermediate precision of 8.2% is excellent. In summary, these data demonstrate the suitability of the GLP-1R qualified bioassay for use in RP assays for potential GLP-1R agonists.
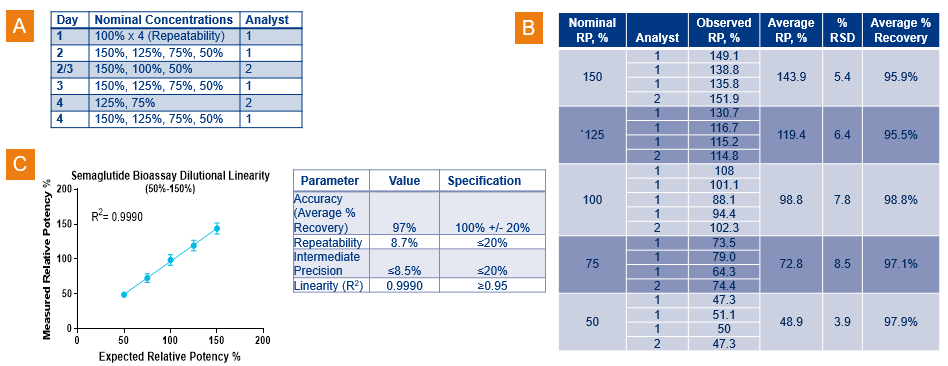
Figure 3. Qualification data for the cAMP Hunter Semaglutide GLP-1R Bioassay Kit. A. Five nominal concentrations of Semaglutide were evaluated over a range of 50-150% with repeatability (four runs) assessed at the 100% NCs by a single analyst. Intermediate precision incorporated several sources of variability, including two analysts over multiple days and two lots of bioassay target cells. B. Table shows observed relative potency values obtained for all tested NCs. C. Results obtained from this qualification study indicate excellent accuracy, high repeatability, intermediate precision, and dilutional linearity for this Semaglutide GLP-1R bioassay.
Conclusions
The development of the RTU GLP-1R bioassay kit has been qualified with Semaglutide and offers a seamless homogeneous format to interrogate the activation of GLP-1 receptor via ligand binding for potency testing in QC lot release programs. Moreover, the cAMP Hunter bioassays are MOA-reflective, functional, cell-based assay kits showing lot-to-lot consistency and offered as both ‘target-based’ and ‘qualified’ Kits. These bioassay kits are qualified with several drugs such as Byetta (BYETTA® is a registered trademark of Amylin Pharmaceuticals LLC. used under license by AstraZeneca Canada Inc), Victoza (Victoza® is a registered trademark of Novo Nordisk A/S), and now, Ozempic , with the capability of measuring ligand mediated cAMP accumulation.
Resources and Related Products
- Semaglutide GLP-1R Bioassay Kit (Catalog No. 95-0062Y2)
- Related Products: Exendin-4 Bioassay kit, GLP1 (7-37) Bioassay Kit
- cAMP Detection Solutions for Small Molecules & Biologics (discoverx.com/camp-assays)
- Bioassay Kits (discoverx.com/bioassays)
