ACCELERATING CYTOKINE DRUG DISCOVERY AND QC LOT RELEASE TESTING WITH QUALIFIED, FUNCTIONAL CELL-BASED ASSAYS
Cytokines and their receptors have emerged as major drug targets for inflammatory and autoimmune diseases, allergies, and, more recently, in cancer immunotherapies (1). There has been significant growth in the last decade in the number of cytokine-based clinical and preclinical development trials. Key reasons for this growth include an advanced level of understanding of the immunological functions of cytokines, a renewed interest in the anti-tumor properties of cytokines, and the availability of improved drug engineering options.
Important improved options for drug candidates under clinical development include new therapeutics and many known molecules repurposed for novel indications or new targets. In addition, novel approaches to protein engineering have produced intelligently designed fusion proteins that increase the half-life and target cytokine activity to the tumor microenvironment or the desired effector immune cells. Conversely, the immunosuppressive role of cytokines is considered when targeting blockage of signals by antagonistic antibodies, small molecules, or siRNAs (2).
Why Are Functional Cell-Based Assays Critical for Cytokines Therapeutics?
Functional cell-based assays can serve as critical tools for cytokine therapeutic discovery and characterization through development and quality control (QC) lot release testing. Assays targeting specific cytokines are derived from stable cell lines that maintain the same mechanism-of-action (MOA) of the cytokine as that of the cell line to produce highly consistent data with excellent assay accuracy and precision — an assay design technology of DiscoverX Ready-to-Use (RTU) kits. The benefit of the DiscoverX RTU kits is that they are MOA-reflective assays that are simple to work with, as well as robust, and flexible, allowing for reduced assay variabilities and increased assay throughput.
Another benefit of our assay-ready formats is that they address both short-term and cell-line evaluation needs to anchor early discovery phase applications. Plus, DiscoverX’s RTU kits are carefully crafted for QC lot release testing programs and designated as “Bioassays” that are qualified using marketed drugs, including those specific for cytokines.
Eurofins DiscoverX’s bioassays demonstrate excellent linear potency and data quality applicable for GMP testing programs, thereby decreasing assay development and validation times. See the streamlined workflow schematic for the DiscoverX bioassay development for targeting therapeutics including cytokines below in Figure 1.
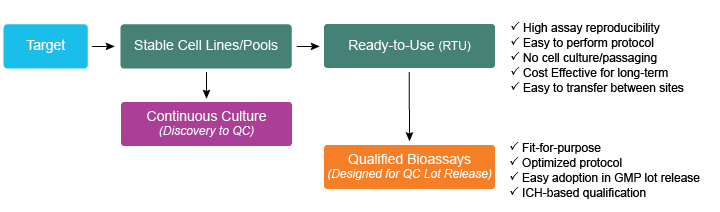
Figure 1. Flow schematic of Eurofins DiscoverX’s bioassay development. Major drug target classes including that of cytokines are identified for developing robust and high throughput assays. Assays targeting these are derived from stable cell lines maintaining the same MOA as that of the cell line. Target-based bioassays reflective of the MOA provide phase-appropriate solutions right from continuous culture to bioassays optimized as RTU kits, and further qualified with standard qualification parameters as Qualified bioassays. GMP = Good Manufacturing Practice. ICH = International Council for Harmonisation
In summary, cytokines drug discovery and development can be accelerated when using Eurofins DiscoverX’s functional bioassays, as they:
- Reflect cytokine’s physiological MOAs (such as dimerization, downstream signaling, and signaling pathway reporters)
- Provide homogenous read-outs with all required reagents included
- Allow GMP QC lot release potency and stability testing of cytokine therapeutics
These DiscoverX RTU bioassays are optimized following systematic qualification studies to meet regulatory expectations (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use and United States Pharmacopeia guidelines). During these qualification studies, key attributes desired in functional bioassays are evaluated, such as accuracy, precision, linearity, range, specificity, robustness, and stability-indicating properties. These attributes are deemed critical in any bioassay when measuring drug potency and stability during drug manufacturing and release under QC conditions.
How Can Cell-Based Assays Be Used to Assess Cytokine Therapeutics?
Cell-based assays provide functional responses such as receptor dimerization that reflects cytokine’s MOA. Plus, when the assays are optimized into a ready-to-use format, qualification for potency and stability testing can be achieved in a timely manner. Learn more about how it’s achieved:
-
IL-2 Dimerization Assay Using the Versatile Enzyme Fragment Complementation Platform
The cytokine interleukin (IL) IL-2 is a 15kDa glycoprotein produced primarily by activated T lymphocytes. The receptor for IL-2 is composed of three subunits: the IL-2 receptor α (CD-25); β (CD-122); and γ (CD-132). The IL-2 receptor γ and β chains form an intermediate-affinity receptor that is the signal transducing subunit of the receptor. The α receptor subunit is a low-affinity receptor by itself, but in combination with the β and γ chains, it forms the high-affinity IL-2 receptor. Likewise, the IL-15 receptor comprises the IL-2 receptor β and γ subunits (3). The following Eurofins DiscoverX Enzyme Fragment Complementation (EFC) based assay demonstrates how IL-2 dimerization and association of IL-2 subunits are measured.
In the PathHunter® receptor dimerization assay (Figure 2.), the IL-2β receptor subunit is tagged with an enzyme donor (ED) [small fragment of EFC β-galactosidase (β-gal) enzyme], while the IL-2γ receptor subunit is tagged with an enzyme acceptor (EA) (larger complementary fragment of β-gal enzyme). Upon agonist (IL-2 or IL-15) treatment, the two-receptor subunits dimerize, forming an intermediate, high-affinity receptor representing the signal transduction function of IL-2. Concurrently, this receptor dimerization causes the fusion or complementation of two fragments, ED and EA. The fully functional β-gal enzyme can now hydrolyze a chemiluminescent substrate producing a quantitative signal that can be captured in a luminometer.
Ultimately, this assay produces a quantifiable gain-of-signal sigmoidal response as a measure of IL-2 receptor dimerization. In a similar fashion, the same assay can be used to quantify an inhibitor response from an anti-receptor or anti-ligand molecule against IL-2. For example, when an antibody blocks the IL-2 receptor, a loss of signal response can be measured with a quantifiable inhibitory signal, thus highlighting the flexibility for PathHunter cell-based assays.
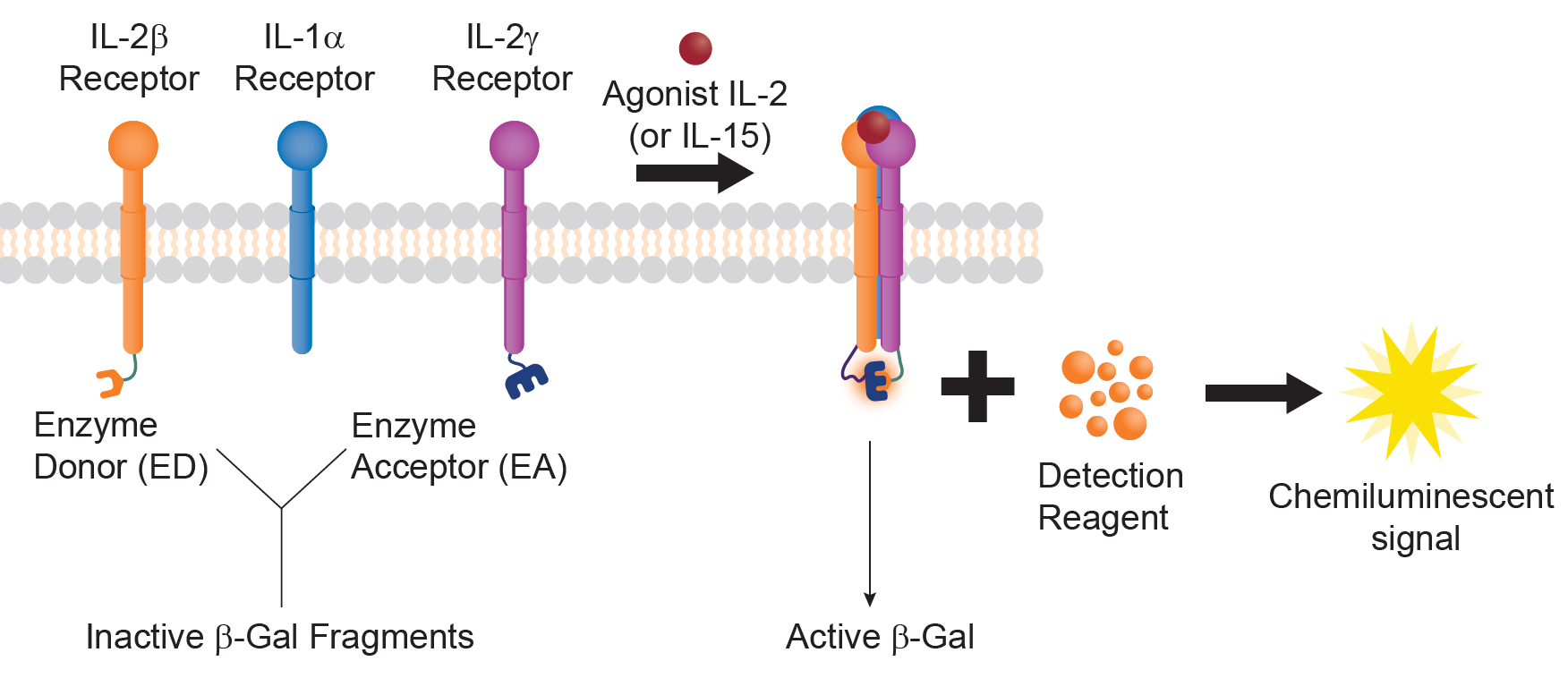
Figure 2. Receptor dimerization assay with the EFC technology. IL-2 subunits are tagged with β-gal EA and ED fragment, respectively. Upon agonist treatment, the two subunits dimerize resulting in complementation of the two fragments to make a functional β-gal enzyme, whose activity can be measured in a luminometer.
With the IL-2 dimerization assay as an example, several other assays for different interleukin receptors across multiple families include IL-1, IL-7, IL-12, IL-15, IL-17, IL-23, and many others have been successfully implemented with the EFC platform. These assays display complete dose-response gain-of-function sigmoidal curves with large signal-to-background ratios that indicate robust assay windows and high reproducibility.
-
Qualification of Cytokine Bioassays for Potency Testing
Qualified Bioassays using relevant marketed drugs display excellent linear potency. For example, the Tocilizumab (IL-6 receptor antibody) bioassay demonstrates high accuracy, precision, and linearity. In addition, precise potency determination of other cytokines targets such as TNFα, IL-1 and GM-CSF via clinically relevant drugs, such as Adalimumab, Anakinra, and Sargramostim, respectively, has helped with bioassay qualifications (Figure 3.). A high degree of accuracy and exceptional linearity suggests that these bioassays can measure relative potency with high confidence.
Overall, Eurofins DiscoverX’s qualified bioassays (including that of cytokines) have primarily supported the following:
- Qualification and validation of hundreds of targets
- Effortless and routine transfer to other sites
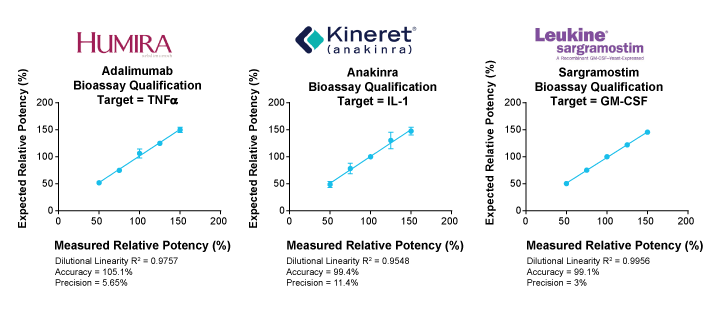
Figure 3. Cytokines bioassay qualification results using key registered drugs. Humira® (AbbVie Inc.), Kineret® (Sobi);, and Leukine® (Partner Therapeutics), all targeting cytokines TNFα, IL-1, and GM-CSF, respectively, and all displaying high degree of relative potency backed by high level of dilutional linearity and accuracy.
Conclusions
Many human cytokines are considered as hot targets for therapeutic development, particularly with a greater variety of cytokine drugs hitting preclinical development and regulatory stature. The variety of cytokine drugs includes, but are not limited to, modified agonists and engineered or modified cytokine drugs such as pegylated IL-2 offered with or without checkpoint inhibitor antibodies (the latter being highly beneficial against a wide range of cancers). As the assortment of cytokines-based targets continue to expand the landscape of cytokine therapeutics at a rapid pace, it is imperative to use specific functional assays that can accelerate cytokines-based drug discovery through multiple stages of the biopharmaceutical development pipeline.
Employing target-specific, qualified bioassays in cytokines drug development programs can help demonstrate not only robust assay performance sensitive to measuring manufacturing consistency, but also the amenability to conduct QC Lot release testing at various sites and correlated measurements with clinical outcomes of the cytokine drug candidate.
Learn more about Cytokine Functional Assays at DiscoverX.com to access functional cell-based assays for over 80% of human cytokines, including for IL-1, IL-2, IL-6, IL-12, IL-7, IL-15, TNFα, GM-CSF, and more.
References
- Sylvia L, Kim M. Cytokines in cancer immunotherapy. Cancers. 2011, 3 (4): 3856-3893.
- Berraondo P, et al. Cytokines in clinical cancer immunotherapy. British Journal of Cancer. 2019, 120 (1): 6-15.
- James V, Talal C. Immune dysregulation leading to chronic autoimmunity. Stiehm’s Immune Deficiencies. Academic Press. 2014: pp. 497-526.
Resources
- Cytokine and interleukin receptor assays
- Ready-to-use, cell-based bioassays for comparability and QC lot release testing
- Functional cell-based assays to support COVID-19 drug discovery through QC lot release programs
- Signaling pathway reporter assays for understanding therapeutic MOAs
- EFC platform for interrogating biomolecular reactions
