Targeting Pro-Inflammatory Cytokines for COVID-19 Therapy: Cell-Based Assays for Accelerated Drug Discovery
The COVID-19 pandemic has left us racing against time for remediation. As a result, we have witnessed hundreds of therapeutic strategies emerge globally in the last several months. Among several noteworthy approaches is the targeting of pro-inflammatory cytokines such as IL-6, IL-1, TNFα, GM-CSF, etc. An uncontrolled and dysfunctional release of these cytokines after viral infection causes cytokine release syndrome (CRS), a condition linked to mortality in COVID-19 patients. Therefore, several new and existing drugs targeting these cytokines are in development for COVID-19 treatment. However, this requires robust assays that can accelerate the development and market-release of such therapeutics, but such assays have repeatedly proven to be challenging to develop. From cell line development and characterization to assay qualification and implementation, the process of assay development can take anywhere from nine months to over a year, thus impacting the timeline of a drug’s release into the market. This blog briefly discusses different cell-based assays that are suitable for cytokine-related COVID-19 drug discovery, for both biologics as well as small molecules.
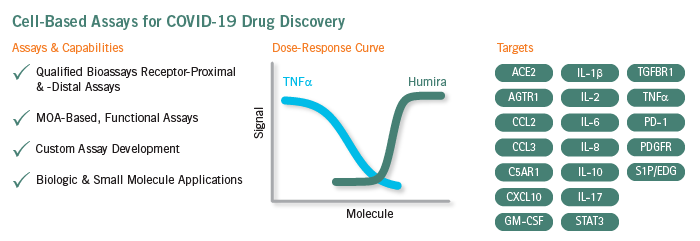
Qualified Bioassays for COVID-19 Drug Discovery
To address the challenges discussed above, Eurofins DiscoverX offers a comprehensive menu of ready-to-use, qualified bioassays for drugs targeting the key cytokines. Each of these bioassays reflects the drug’s mechanism of action (MOA) and is qualified with specific innovator drugs, e.g., tocilizumab (Actemra®), anakinra (Kineret®), adalimumab (Humira®). During qualification, each bioassay is rigorously tested to ascertain a high degree of assay accuracy, precision, linearity, and reproducibility to ensure these are fit-for-purpose for potency and stability testing. Thus, such qualified bioassays that would otherwise take over a year to develop are readily available for implementation in drug development programs.
In addition to being fit-for-purpose, these assays are also easy to implement. Derived from stable clonal cell lines, the bioassay cells have been optimized to a ready-to-use format. The cells only need to be thawed, plated, and incubated with the relevant reagents provided in the kit for 24-48 hours before reading the results. Such qualified bioassays with simple, homogenous protocols not only reduce assay run time, but also enable seamless global method transfer.
Signaling Reporter Assays for COVID-19 Drug Discovery
Interrogating therapeutic activity through downstream signaling events in addition to upstream or receptor proximal-based assays is an excellent way to gain a comprehensive understanding of the therapeutic. Receptor distal-based assays take advantage of reporter proteins to measure downstream transcriptional events after the ligand/drug activates the cell surface receptor. Like other Eurofins DiscoverX cell-based assays, these signaling reporter assays are rapid, MOA-reflective, easy-to-run and amendable to high-throughput format for increased efficiency. For COVID‑19 drug discovery focused on the IL-6 pathway, one can imagine using both types of cell-based assays to evaluate the potency of the antagonist therapeutic Tocilizumab. Specifically, the IL-6 receptor proximal-based PathHunter® Tocilizumab Bioassay to evaluate drug-induced receptor heterodimerization and receptor distal-based the PathHunter HepG2 STAT3 Pathway Reporter Assay to measure the activation of STAT3 as a consequence of receptor activation. By assessing both upstream and downstream drug-induced, receptor responses, a complete description of the drug molecule’s MOA is revealed.
COVID-19 Assays for Small Molecule Drug Discovery
Another active area of COVID-19 therapeutic research is the development of JAK (Janus kinase) inhibitors. Known for transducing cytokine-mediated signals via the JAK-STAT pathway, JAK is a target of interest because its inhibition is purported to have anti-inflammatory as well as anti-viral effects. Small molecule inhibitors such as ruxolitinib and baricitinib, among several others, are currently in clinical trials for the treatment of COVID-19. Eurofins DiscoverX offers cell-based functional assays that evaluate the activity of such drugs. Examples include the PathHunter U2OS CSF3R-JAK1 Functional Assay and JAK3 Activity Assay. Similar to the bioassays and signaling reporter assays, these are easy-to-use, amenable to high-throughput, and provide robust assay windows.
Conclusions
Whether it is a biologic or small molecule targeting COVID-19-related cytokines, Eurofins DiscoverX strives to support you through its expertise in cell-based assay solutions. Qualified, MOA-based ready-to-use bioassay kits are designed to provide excellent results with relative ease, thus saving you valuable time. Orthogonal to these tools are the signaling reporter assays that quantify downstream cellular signaling events. Additionally, when an off-the-shelf solution is not available, Eurofins DiscoverX custom assay development capabilities enable you to pursue your drug target of interest with confidence.
Actemra® is a registered trademark of Chugai Seiyaku Kabushiki Kaisha Corp., a member of the Roche Group; Kineret® is a registered trademark, licensed by Swedish Orphan Biovitrum AB and marketed by Sobi, Inc.; and Humira® is a registered trademark AbbVie, Inc.
Resources
