USE OF AUTOMATED FUNCTIONAL, CELL-BASED BIOASSAYS
Importance in Drug Discovery & Development for Quality Testing Programs
Cell-based assays have emerged as crucial tools for basic and applied research. They have underscored their growing presence and importance in recent times, particularly in biologics and small molecule discovery and development (1).
Cell-based assays have been implemented in key stages and modalities such as screening, target engagement, receptor modulation, and cytotoxicity to better aid with drug discovery and development workflows. Eurofins DiscoverX strives to minimize the complexity of assay protocols to achieve high assay reproducibility. This is especially important in quality-controlled (QC) lot-release potency testing programs. Correctly developed cell-based potency assays provide this need of precision, accuracy, and reduced assay variability, all directed towards scaling up capabilities and/or method transfers.
In addition to being at the forefront of reducing typical bioassay development timelines with qualified bioassay programs, Eurofins DiscoverX is poised to minimize the effects of dynamic factors impacting cellular stability including targets and reagents used in bioassays. This is done by integrating automation (e.g., through Tecan’s Fluent® Automation Workstation) into our cellular workflows, thereby also minimizing human error and time. By automating PathHunter® cell-based bioassays, we have demonstrated the retention of functionality consistently reflective of the following:
- Therapeutic Mechanism-of-Action (MOA)
- Stability
- Dilutional Linearity
- Reproducibility
- Assay Throughput
- Transfer of Assay to other Sites
Automating PathHunter CHO-K1 CNR1 Bioassay
Eurofins DiscoverX’s PathHunter CHO-K1 CNR1 Bioassay based on the well-established Enzyme Fragment Complementation (EFC) technology (refer to discoverx.com/EFC for details) (2) was run on Tecan’s Fluent Automation Workstation (780 System) to analyze β-arrestin recruitment to CNR1, a well-known GPCR (Figure 1) (3). A similar set of experiments was conducted manually and the data was compared to evaluate the efficacy of automation for reproducibility and variability among other parameters.
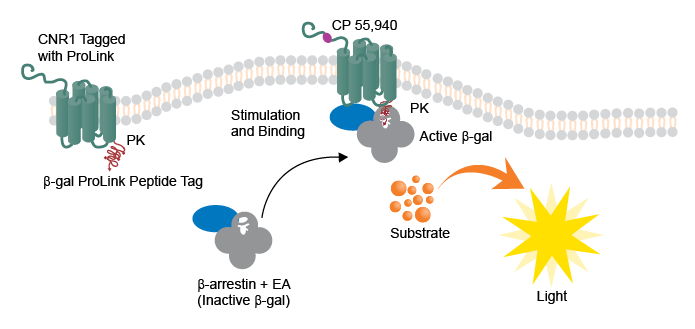
Figure 1. Schematic of the CNR1 bioassay principle.
Briefly, PathHunter CHO-K1 CNR1 [a type 1 cannabinoid GPCR (CB1)] cells were treated with increasing concentrations of agonist (CP 55,940) for 120 minutes at 37°C and 5% CO2. The addition of the agonist resulted in the detection of β-arrestin recruitment to CNR1 via enzyme fragment complementation (EFC) platform. The detection was achieved as a result of complementation of two β-galactosidase (β-gal) enzyme fragments (one of which, a small enzyme donor [called ProLink™ (PK)] fragment fused with CNR1, and the other, a larger enzyme acceptor (EA) fragment fused with β-arrestin) within the cells. The activity of resultant β-gal enzyme (complementation) activity was measured as a chemiluminescent signal using a luminometer following the addition of a chemiluminescent detection reagent (Cat. No. 93-0933). Experiments were performed to assess intra- and inter-day precision for data generated using automation as well as for manual mode (no automation). For detailed protocol showing steps including those involving minimal human intervention vs. automation steps, please refer to the accompanying Appnote.
Results
We have demonstrated reliability and reproducibility when performing a Eurofins DiscoverX Bioassay when performed on a Tecan’s Fluent Automation workstation (Figure 2).
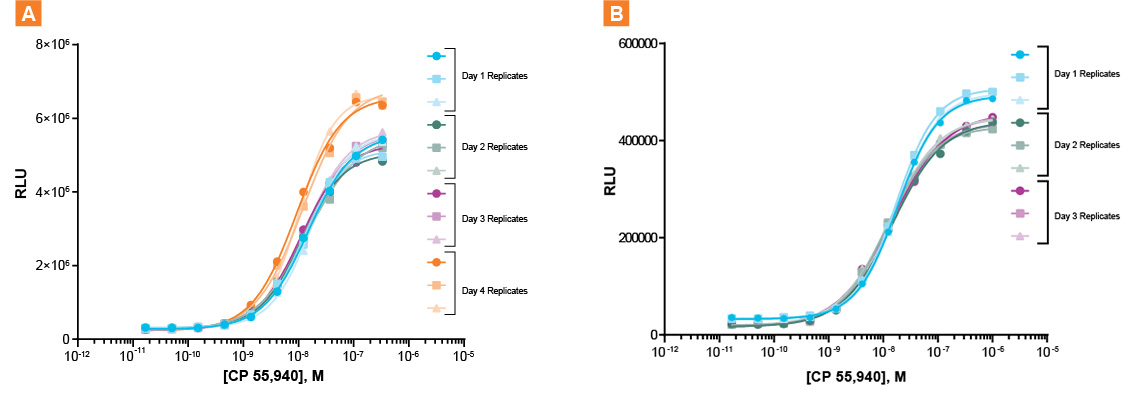
Figure 2. Dose-response experiments conducted using A. Tecan’s Fluent workstation and B. Manual protocols across 4 independent days. RLU = Relative Luminescence Units
Inter-day results show high reproducibility with low variability across replicates when using the automated bioassay. Each replicate dose-response curve across independent experiments shows high inter-day assay precision in EC50 values (Automated- 1.3 e-8; Manual – 1.47 e-8) with an average relative standard deviation (RSD) of 13.5% with the automated bioassay compared to 17.5% with the manual method.
Conclusions
- Performance of the PathHunter CHO-K1 CNR1 Bioassay was robust and showed highly reproducible results on the Tecan Fluent Automation system
- The results from the assays run using automation were remarkably similar compared to the manually executed assays run by an experienced QC scientist with several years of experience, with the inter-day precision for the automation data having a slight edge over the manually generated dataset.
- Low replicate variability across intra-day and inter-day experiments corresponds with high precision and repeatability.
The bioassay format is ideally suited for automation, demonstrating high reproducibility and low variability. It is representative of a multitude of other assays already available in Eurofins DiscoverX’s cell-based assay portfolio. Further, this bioassay performed robustly out-of-the-box with minimal optimization needed for running on Tecan’s Fluent automation system; making it more amenable for technology transfer and use for off-site contract research organizations (CROs), and contract development and manufacturing organizations (CDMOs).
Overall, the use of automated functional, cell-based potency assays for drug discovery and development will not only aid in obtaining highly reproducible and reliable data, but also reduce time and cost to implement within any given QC-lot release program.
References
- Schneider, G. Automating drug discovery. Nat Rev Drug Discov 17, 97–113 (2018). https://doi.org/10.1038/nrd.2017.232
- EFC (Enzyme Fragment Complementation Assay Technology)
- β-Arrestin Cell-based Assays and Reagents for Drug Discovery
Resources
- Lucia Bruzzone, Venkatesh Chari, Neil Charter, and Gaurav Agrawal. Automating Eurofins DiscoverX Cell-Based Bioassays on Tecan’s Fluent Automation Workstation: Bringing Higher Throughput, Improved Assay Consistency, and Reproducibility for Easy Implementation in Quality Testing Programs. Application Note, Eurofins DiscoverX, October 2021.
- Michael Nosswitz, Venkatesh Chari, Arvind Kannan, Neil Charter, and Gaurav Agrawal. Automating Cell-Based Bioassay on Tecan®’s Fluent Automation Workstation: Measuring PD-1 Signaling. Application Note, Eurofins DiscoverX, September 2022.
- Ready-to-Use Cell-based Bioassays for Comparability and QC Lot Release Testing
