Enabling High-Throughput Screening for Cellular Compound-Target Engagement through InCELL Pulse™
Information on compound-target engagement obtained from traditional in vitro binding assays differs greatly from that in a physiologically relevant model (in vivo). Hence, there has been an increasing trend towards deploying cell-based assays that enable the accurate determination of target engagement in the early stages of hit identification and lead generation. These early stages typically involve screening and profiling of large numbers of compounds, thus demanding methods amenable to high throughput. Cellular thermal shift assays constitute a powerful tool to quantify compound-target engagement within a cell. The underlying principle of these assays revolves around the altered thermal stability of proteins upon binding of compounds or ligands. A native protein undergoes denaturation and aggregation over time when subjected to elevated temperatures, while engagement of the protein with a compound alters its thermal stability. This shift in thermal stability is accurately measured by such an assay and gives information on the tightness and degree of ligand binding.
Typically, these cellular thermal shift assays are limited to use in a low-throughput format, which in turn restricts the number of samples that can be tested in a given time frame. In addition, these methods are tedious even for small numbers of samples. Examples of low-throughput techniques include Western Blot, mass spectrometry, or 96-well plate cell-based assay format. Furthermore, some cellular thermal shift assays require antibodies specific to the target protein for measuring protein levels. While these constraints pose a challenge, it is possible to optimize these assays into a high-throughput (384-well or higher), no-wash format that doesn’t require antibodies or mass spectrometry. Cell-based compound-target engagement assay platforms such as InCELL Pulse™ by Eurofins DiscoverX enable this development. Here, we present two different instances where this InCELL Pulse platform was successfully used to create high-throughput, homogenous, and reproducible assays for efficient screening of target inhibitor candidates.
High-Throughput Dose-Response Cellular Thermal Shift Assay for Screening Inhibitors of SMYD3
McNulty et al. developed a high-throughput dose-response cellular thermal shift assay for screening inhibitors of SMYD3, a histone methyltransferase implicated in the pathology of various cancers. The assay development strategy sought to address two challenges: eliminating the need for all wash and centrifugation steps for assay automation, and using a detection system that is easy-to-use and allows for a homogenous readout. The InCELL Pulse assay platform was found to meet their requirements.
Deploying the InCELL Pulse system in this assay enabled the screening of 123 SMYD3 inhibitors with full dose-response curves. This included inhibitors that bind to the target at the active site or show alternate modes of inhibition. The data generated from these assays correlated to those from the other cellular mechanistic assays and Western Blot target engagement analyses. Furthermore, the broad suitability of InCELL Pulse was tested through use in another assay for screening IDO1 (Indoleamine 2,3-Dioxygenase 1) inhibitors.
Thus, the high-throughput, dose-response cellular thermal shift assay eliminates the need for antibodies, wash steps and complex equipment, and serves as a promising technique in the screening of promising drug candidates.
Read the full paper to learn more about the development of this assay.
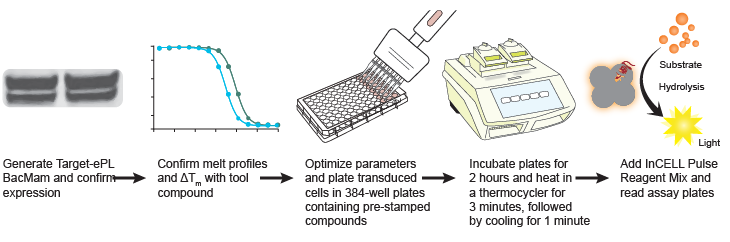
Figure 1. Generic workflow of a high-throughput cellular thermal shift assay using InCELL Pulse™. Source: McNulty DE, et al. SLAS Discovery v23(1): 34-46 (2018).
High-Throughput Cellular Target Engagement Assay for Characterizing SHP2 Phosphatase Inhibitors
Interest in drugs targeting SHP2 for oncology indications and reports on SHP2 allosteric inhibitors called for an assay that would rapidly validate the binding of these candidates with the target. By incorporating the InCELL Pulse platform, Lambert et al. developed a miniaturized, robust, and reproducible cellular thermal shift assay and showed results from screening multiple SHP2 inhibitors. More specifically, this assay was used to quantify the engagement of inhibitors with wild-type SHP2 and E76K—the oncogenic variant of SHP2.
The potencies of various SHP2 inhibitors was measured through this assay. To confirm that the assay functions as it should, the results obtained were compared with those from biochemical assays and in vitro protein thermal stability assays. The potency results were not only in alignment but in addition, the melting temperatures of SHP2 proteins (without – bound inhibitors) were found to match with those from the in vitro protein thermal stability assay. Further confirmation tests deemed this high-throughput cellular thermal shift assay is suitable for the validation and screening of inhibitors targeting wild-type SHP2 and E76K. Read the full paper to learn more about this assay.
For more information on how InCELL Pulse™ works, and our other target engagement assay platforms, visit discoverx.com/incell.
Additional resources can be found at:
- Knowledge-Based Video: InCELL Target Engagement Assays for Drug Discovery
- Webinar: Learn How to Make your Own Compound-Target Engagement, Cell-Based Assays
- Blog post: Discover Critical Challenges and Needs in Cellular Target Engagement
- Blog post: Read About Benefits and Challenges of Do-it-Yourself Cell-Based Assays
- Blog post: Comparison of InCELL Hunter vs InCELL Pulse for Analyzing Compound-Target Engagement
- Application Notes:
- Presentation: Development of High-Throughput Cellular Thermal Shift Assays: Cellular Mechanistic Assays in Early Drug Discovery
