Uses & Advantages of Membrane Preparations for GPCRs
Cellular membrane proteins play important functions in many biological processes. Understanding their roles often requires that these proteins be isolated from their cellular context. This can be achieved by disruption of the cell membrane using various mechanical methods and differential centrifugation¹. Membrane preps generated through such methods result in small fragments of cell membranes expressing large quantities of receptor proteins.
Eurofins DiscoverX Membrane Preparations or Preps are purified membranes derived from stable cell lines that are optimized for high expression levels of the targeted GPCR protein. They are excellent for studying binding affinities of GPCR targets, without the requirement for cell culture.
Membrane Preparations’ Usage
Eurofins DiscoverX Membrane Preps are intended for two main purposes: (1) to screen for ligand binding affinities using a radiolabeled ligand approach; or (2) to screen for GPCR activity using a radiolabeled GTPγS approach. For binding affinities or GPCR activation levels, these can be calculated based on changes in the resulting amount of radioactive signal in the assay. An increase in signal is seen for GTPγS and saturation binding assays, while a decrease in signal is seen for competition binding assays.
Radiolabeled Binding Assays
Radiolabeled ligand studies can be performed using our membrane preps in two formats – saturation binding and competition binding. The differences between these formats are described below.
Saturation Binding
The saturation binding format is achieved by adding increasing doses of radiolabeled ligand in the presence (non-specific binding curve) or absence (total binding curve) of extremely excessive amounts of non-labeled ligand and subtracting non-specific from total binding to get a saturation binding curve. Saturation binding assays can be used in combination with information on IC₅₀ or EC₅₀ from other sources to determine the Kd (dissociation equilibrium constant or binding affinity) of the ligand.
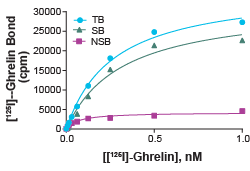
Figure 1: Saturation binding experiment for Ghrelin Receptor (Cat. No. HTS187M). Ghrelin Receptor Membrane Preparation (5.0 µg/well) was incubated with increasing amount of [¹²⁵ᴵ]- ghrelin in the absence (total binding, TB) or presence (nonspecific binding, NSB) of 200-fold excess unlabeled ghrelin. Specific binding (SB) was determined by subtracting NSB from TB. The Kd of ghrelin and Bₘₐₓ (total number of receptors) of the system can be calculated from the specific binding curve.
Competition Binding
Competition binding format involves using one low dose of radiolabeled ligand, and increasing concentrations of non-labeled ligand. This will produce a binding-characteristic-dependent competition curve showing how the amount of radiolabeled ligand-bound decreases as the concentration of non-labeled ligand increases. Competition binding assays can be used to determine the Kᵢ (inhibitory constant) of the test ligand, as well as the IC₅₀ of the unlabeled ligand with respect to the radiolabeled ligand.
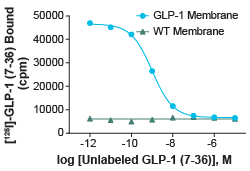
Figure 2: Competition binding experiment for Glucagon-like peptide-1 GLP-1 Receptor (Cat. No. HTS020M). GLP-1 membrane preparation or wild-type (WT) Chem- 1 membrane preparation (Cat. No. HTS000MC1) was incubated with 0.5 nM [125I]-GLP-1 (7-36) and increasing concentrations of unlabeled GLP-1 (7-36), producing a competition curve. Using this curve, Ki and IC50 of the unlabeled GLP-1 (7-36) can be calculated. More than 7-fold signal to background was obtained.
GTPγS Activity Assays
GTPγS assays work by using a radiolabeled non-hydrolyzable GTP analog. This analog is added into the buffer with the membrane preparation. Then the membrane is provided with the known or experimental ligand for the GPCR expressed on the membrane. If the GPCR is activated, the now-available Gα protein will exchange its GDP for GTP. Due to the non-hydrolyzable nature of GTPγS, the Gα protein will then remain permanently bound to the GTPγS. After the removal of excess unbound GTPγS, radioactive detection methods can then be used to determine how much GTPγS remains bound by Gα, and therefore how much of the target GPCR was activated by the applied ligand. This assay is a useful alternative approach to checking agonism vs antagonism and determining potency or rank order of experimental ligands in an easy-to-use format that doesn’t require cell culture steps.
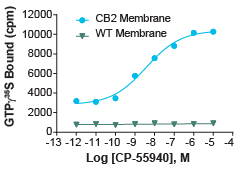
Figure 3: GTPγS assay for Cannabinoid-2 (CB2) Receptor (Cat. No. HTS020M). 5 µg/well CB2 membrane preparation or WT Chem-1 membrane preparation (Cat. No. HTS000MC1) were incubated with 0.3 nM [35S]-GTPgS, 10 µM GDP, and increasing amounts of unlabeled CP-55940. Bound radioactivity was determined by filtration and scintillation counting. An EC50 of 3.7 pmol/mg and signal to background of > 3.4 fold were obtained from this curve.
.
Eurofins DiscoverX Membrane Preps Validation
The Eurofins DiscoverX membrane preps have been tested for and provide reference data in either GTPγS or binding assay applications, not both. They have also been validated with radioligands and not been specifically tested using fluorescent or other ligand labeling methods. These membrane preps are intended for filtration-based ligand labeling strategies and were not validated using scintillating plates or bead-bound methods. Attempting to use these other strategies will require some optimization. Overall, if you’re interested in studying binding affinities of GPCR targets, without a requirement for cell culture, pre-prepared membrane preps are an excellent assay tool to have in your toolbox.
Resources
Access Eurofins DiscoverX membrane prep for GPCRs and Ion Channels at discoverx.com/membrane-preps.
References
- Roy A. Membrane preparation and solubilization. Methods Enzymol. 2015; 557: 45-56. doi:10.1016/bs.mie.2014.11.044
